mo diagram of oxygen
We have 9 Images about Explain formation of O2. - At this point try making the MO diagram for oxygen on paper using the LCAO-MO method an example is given in section 10-3 of the book.
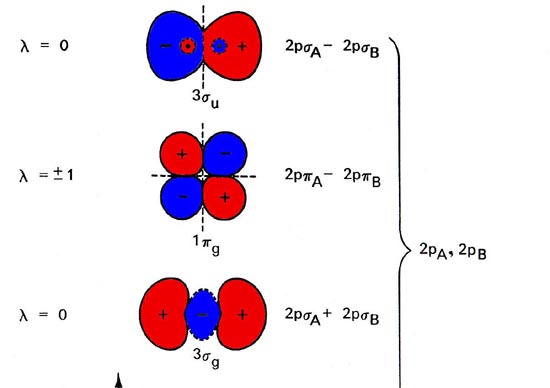
Molecular Orbitals Molecular Orbitals For Homonuclear Diatomics
MO Diagram of O2 Molecular Orbitals Oxygen 22346 views Mar 26 2014 142 Dislike Share Save chemistNATE 217K subscribers How to draw the molecular orbital diagram.
. This adds up to the explanation of the molecular orbital diagram of SO2. It has two unpaired electron in its bonding molecular orbital. Molecular orbital diagram of two singlet excited states as well as the triplet ground state of molecular dioxygen.
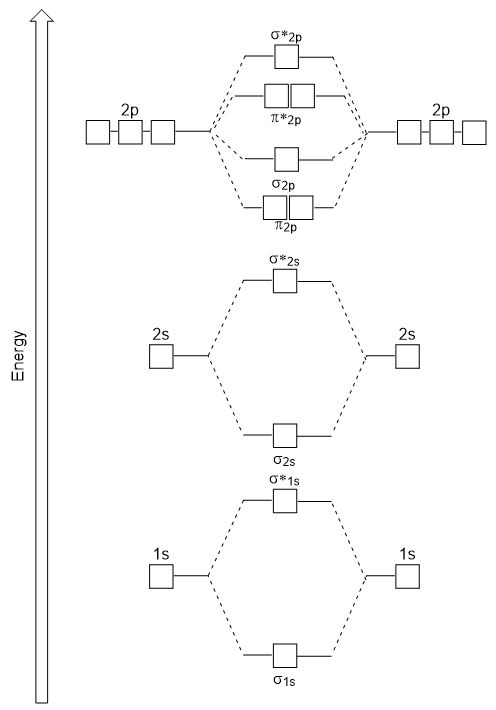
Include the core 1s electrons in your diagram. MO Diagram - A molecular orbital diagram also known as a MO diagram is a qualitative descriptive tool used to explain chemical bonding in molecules using molecular orbital theory. MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine.
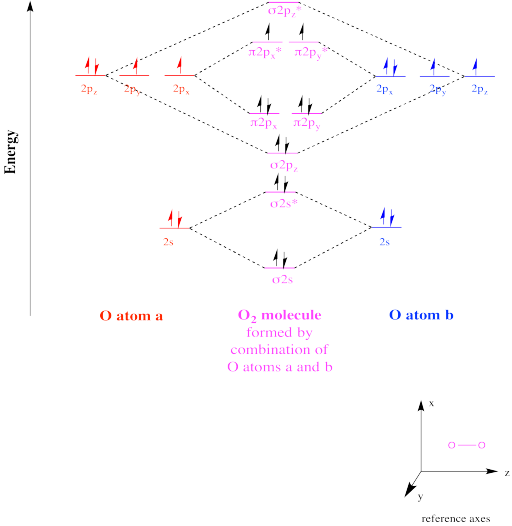
The atomic number of Oxygen is eight and electronic configuration is 1s2 2s2 2px2 2py1 2pz1. Oxygens paramagnetism is explained by the presence of two unpaired electrons in the molecular. Molecular Orbital MO Diagram for O2 - 65125 views Jul 30 2020 When two oxygen atoms overlap the sigma 2p molecular orbital is LOWER in energy than the pi 2p.
O2 is more stable than O2. Materials and Molecular Research Division of Lawrence Berkeley Laboratory University of California 94720 Berkeley California. In the case of O 2 17 electrons.
Because According to molecular orbital theory O 2 has 15 electrons it has one electron in antibonding orbital. 1 Δ g singlet oxygen first excited. Asked Oct 17 2020 in Chemical Bonding by Rajan01 467k.
The F 2s is nonbonding. - At this point try making the MO diagram for oxygen on paper using the LCAO-MO method an example is given in section 10-3 of the book. If you are searching about Explain formation of O2 molecule with the help of MO diagram pls youve came to the right place.
Firstly we now add our newly acquired. Similarities between Sulfur and Oxygen atoms Both O and S have the same outer electric configuration of ns2 and. Each oxygen atom contributes six electrons to O2 molecule from its valance shell.
HF nb σ σ Energy H 136 eV 1s F 186 eV. From left to right the diagrams are for. Oxygen molecule is paramagnetic.
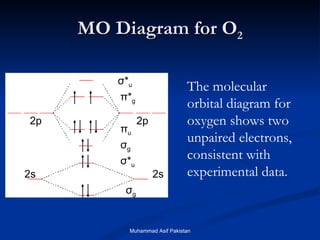
It also explains the bonding in a number of other molecules such as. MO theory provides an explanation of chemical bonding that accounts for the paramagnetism of the oxygen molecule. The molecular orbital energy diagram for O 2 predicts two unpaired electrons.
Reactivity Oxygen Reduction

Using The Mo Diagram Of No Calculate The Bond Order Compare It To No Socratic
K Hu5w3hntymym

Draw A Molecular Orbital Diagram Of N2 Or O2 With Magnetic Behavior And Bond Order
Relativistic Coupled Cluster Calculations On The Dioxygen Molecule Dirac 19 0 Documentation
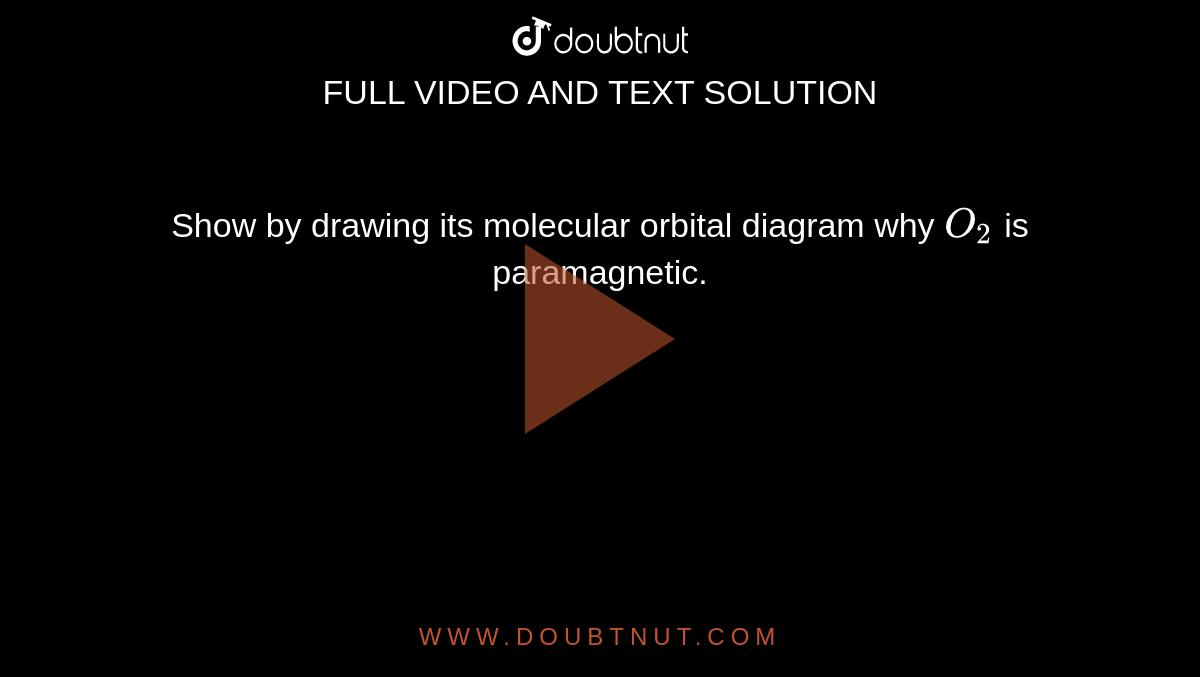
Draw The M O Diagram For Oxygen Molecule Calculate Its Bond Order And Show That O 2 Is Paramagnetic
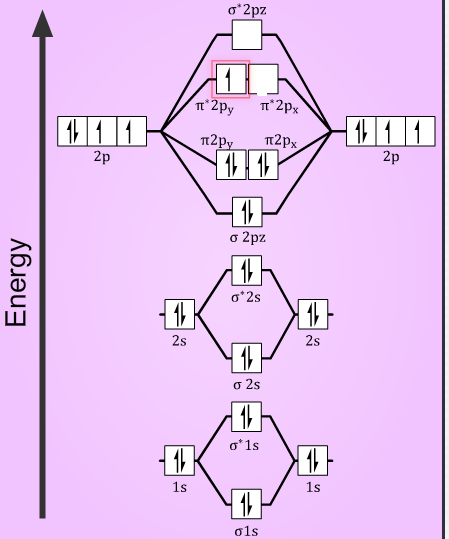
More Stable Among O2 And O2 Chemical Bonding And Molecular Structure Chemistry Class 11
What Is The Molecular Orbital Diagram For Oxygen Quora
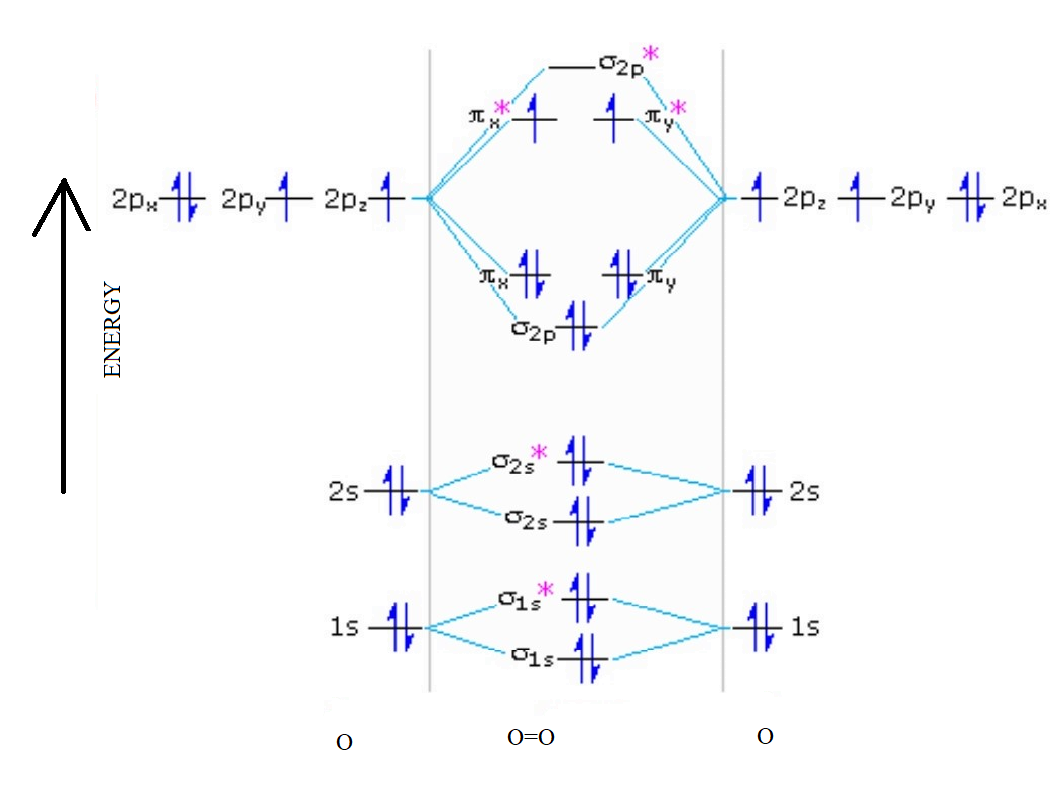
Explain The Formation Of O 2 Molecule Using Molecular Orbital Theory

How Many Unpaired Electron Does O2 Molecule Have
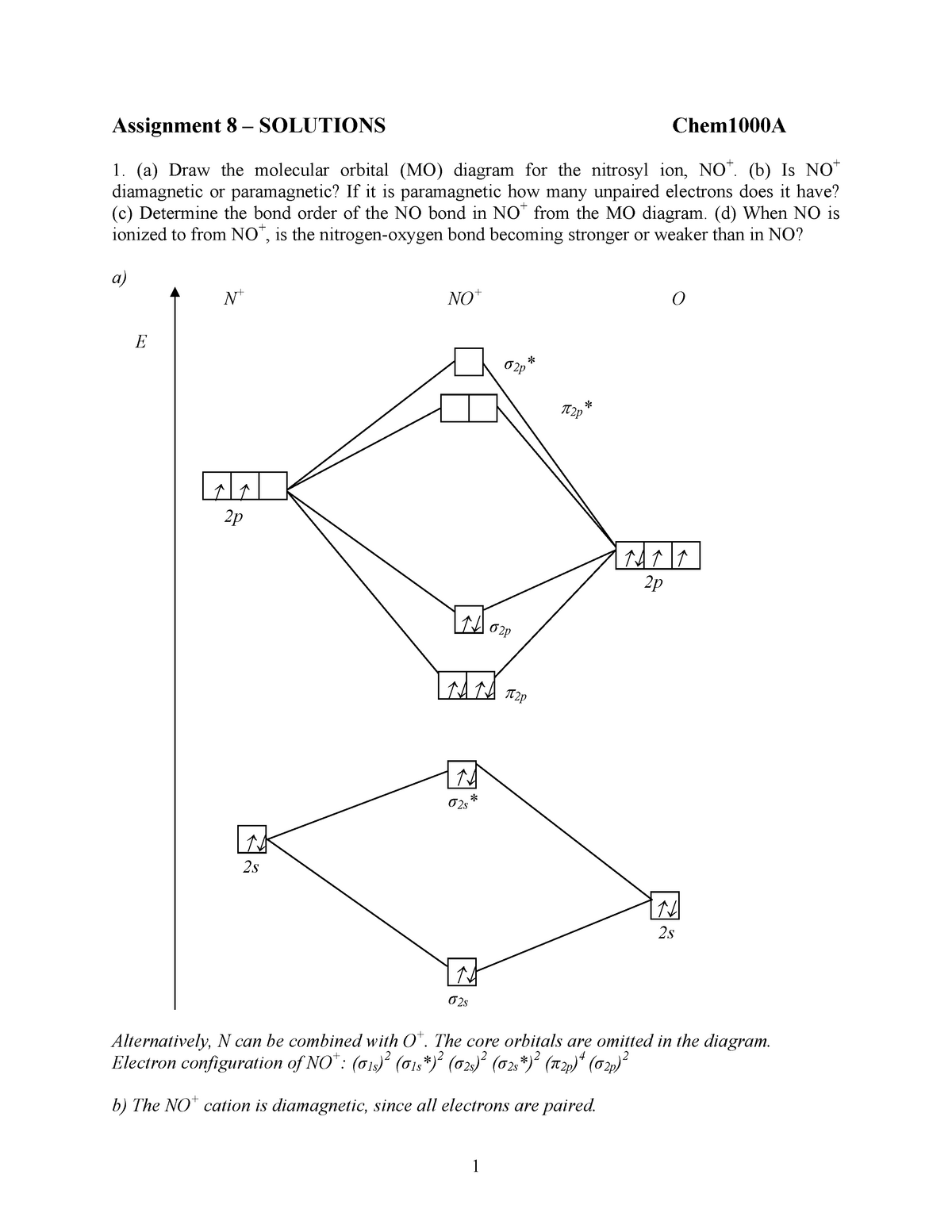
282309837 Assignment 8 Solutvions Assignment 8 Solutions Chem1000a A Draw The Molecular Studocu

13 Molecular Orbital Diagram Of Oxygen Molecule Reproduced From Ref 58 Download Scientific Diagram
Solved 1 A Molecular Orbital Energy Diagram For Co2 Is Chegg Com

Solved Sopling Earning Complete This Valence Chegg Com
Chem1902 Oxygen

Molecular Orbital Diagram Wikipedia
Why Is O2 Paramagnetic Socratic